The process of tanning is the midpoint and the basis of leathermaking. Tanning is the stabilization of the collagen structure of the hide, using natural or synthetic chemicals. The stabilization is mainly an increase in resistance against water and it leads to a restricted swelling.
Resistance against water means, that tanned material can no more underwent changements, which are caused by an aqueous medium: putrification, swelling and drying off to an inflexible solid mass. Tanning also leads to a changement of the appearance and of the handle or feel of skin or other kinds of connected tissues. The object of converting pelt into leather by tanning is to
These effects are achieved by cross-linking the collagen chains with various tanning agents. A variety of chemical agents for tanning is available, with chrome being the most commonly used.
2.1 Tanning with Metal Salts
Of greatest importance is chrome tanning with trivalent chromium compounds. But tanning can be done also with aluminum salts, known and applied for thousands of years. Zirconyl, titanyl and uranyl salts also have tanning actions, although known for half a century. Other tannages included copper salts and the salts of rare earths.
Many different tannages are possible, to meet the demands of the leather industry. However, screening the properties of the leathers produced from different metal salts shows, that most of them have shortcomings or disadvantages, in comparison with leather tanned with chromium compounds.
2.1.1 Chrome Tannage
The majority of leathers today are chrome tanned. This is a consequence of the easy processing, the broad achievability and the unlimited access to Cr-tanstuffs and the excellent properties of the chrome tanned leather. One of its extraordinary properties is the boilfastness.
Modern single-bath chrome tanning with trivalent chromium sulfate is conducted by adding commercially produced powder products to the tan drum. Pickling invariable precedes the tanning step, because of the need to bring the acidity of the pelt to the required level. This acidity is vital for the subsequent tanning procedure. Pickling and tanning nowadays are carried out in a combined step.
Chrome tanning involves:
Characterizing the completion of chrome tanning is to produce boil resisting leather. Most of the time is taken up in building stable complexes. Complex formation is not a fast spontaneous reaction as is, for example the diffusion of chromium compounds into interior of the fibrils.
Reaction through a 1000 ┼ thick fibril within the fiber structure takes up to an hour. Split pelts, less than 3 mm thick, is usually colored through by chrome within 30 minutes. Very thick, full substance, unsplit bull hides (perhaps 10 mm thick) are often penetrated very slowly, can take almost one day to make wet blue.
It is characteristic of these reactions that they approach equilibrium slowly, so that complete exhaustion never happens. If about 100 % float is used with an offer of 2,5 % Cr2O3, maximum exhaustion is about 80 %. Use of shorter float and lower chrome offer promotes efficiency.
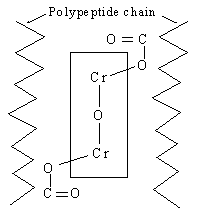
The location of the reaction sites of the chrome are the carboxylgroups of the collagen. Collagen molecules are arranged parallel shown very schematically in figure 6. All possible spaces can be reached by the reactive groups for crosslinking with chrome.
The cross-linking reaction between skin substance and the chrome tanning agent is shown as schematic in figure 5. The cross linking is effected by principal valences through coordinative bonds (complex bonds) with the COOH groups of the collagen.

2.1.2.- Tanning with Aluminum Salts
It is well known that aluminum salts do not tan to produce stable leather in the same way as chromium. Nevertheless, aluminum salts are used for some special purposes: for glacÚ kid, for gloves, in the fur industry and to some extent for suede leather.
Aluminum tannage can help produce brilliant coloration and in the case of suede, gives a fine, short nap. Generally, aluminum salts lead to more firmness or to a more compact leather structure. Aluminum salts can be used in combination with chrome, to substitute part of the chrome offer. For this purpose aluminum tannage is very useful, because it tans fully reversible, the aluminum salts can be stripped off with acid.
Finally, aluminum salts form very stable tanning compounds with polyphenolic tannings. Because of this high stability, this combination can completely substitute chromium.
2.1.3.- Zirconium tannage
Zirconium tannage has been developed for practical use, since its introduction in the early thirties. But the use of zirconium has risen more or less constant though not high level. The limited usage is due to the higher price of the zirconium tanning salt and the particular plumpness of the zirconium tanned leather. Substitution of chrome by zirconium is not possible due to both of these factors
2.1.4.- Titanium tannage
Titanium salts were developed later than zirconium salts, but the chemistry is similar in many respects. Its later development is a reflection of the availability of the titanium salts. The main interest in using titanium salts, is the substitution of chrome.
However, it was evident that effectively no chrome can be saved, if the leather produced has to be boilfast and if the character of the leather is to be comparable to that of chrome leather.
2.1.5.- Iron tannage
Iron tannage has been known as long as chrome tannage but industrial tannage has however been very limited. This is due to many properties of the iron salts in tanning. All iron tanned leather is drawn to a greater or lesser extent and it is sensitive to vegetable polyphenolic tannins, forming black inks, which discolored leather can be obtained, with different shades of black; e.g. deep black with chestnut, gray with mimosa.
Furthermore and importantly, the leather becomes boilfast. Iron tanned leather has a heavy character. It is vulnerable to oxidation, which may lead to deterioration upon ageing; this is certainly the case, but it requires a long time and the reaction is not as fast as has been supposed hitherto.
2.2.- Tanning with Aldehydes
So far as leather making in concerned aldehyde tanning is not capable to bring about a full tannage with a broad spectrum of properties of leather sellable on the market. That is also true for the tannage with glutaraldehyde, which is compound with the best tanning capacity. The basis of the tanning properties of this compounds is the covalent reaction with the aminogroup, univalently, divalently and also multivalently, which leads to crosslinking.
2.2.1.- Glutaraldehyde tanning
Glutaraldehyde is used since some decades for modification and stabilization of proteins and for tanning. The tanning effect is much more effective than with other aldehydes, e. g. formaldehyde. Glutaraldehyde tans well also in acidic medium. But also with glutaraldehyde the taneffect is better in alkaline medium. The increase of shrinkage temperature is much more faster than with other aldehydes. The leather tanned with glutaraldehyde has a special character. It is specific light and usable for garments and furs. It becomes very porous and as aldehyde tanned product, it is wash and sweat resistant as more as the pH increases. The reaction of glutaraldehyde with collagen takes place better in weak alkaline aqueous medium.
2.3 Vegetable Tannage
Many polyphenolic water soluble plant compounds leached from shredded wood, bark, leaves and roots are able to penetrate raw hides or pelts and bind to collagen fibers. The resulting leather may have a final tannin content of as much as 50 to 95 % by weight with respect to the protein content (called degree of tannage).
As a result of the very slow penetration of these large molecules the saturation of the hide with these large amounts of tan stuff takes much more time than with other tannages that are performed with much smaller tanning molecules. Tannin solutions are naturally weakly acidic, with pH values of between 3 and 6, due to a number of acidic carboxyl groups on the molecules relative to hydroxyl groups.
Pelts do not swell in natural tan solutions. Swollen pelts from the alkaline beamhouse regain their natural unswollen state in these solutions very slowly. Hide proteins on the surface of the hide react rapidly with fresh vegetable tannins, causing a constriction of the protein structure. This effect is the astringency of tannin solution. This surfacial reaction slows the diffusion of the tannins into the center of the hide and results in the first tanning stage in uneven tanning of the inside and outside.
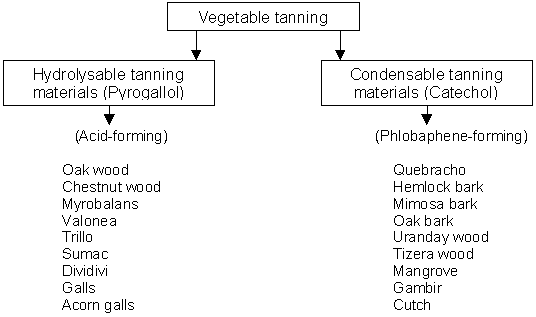
The reaction between hide proteins and natural polyphenols is dependent on the acidity. The principle attraction between protein and tannin is based on hydrogen bonding and dipole interactions. The speed of binding is rapid and is quite firm under acidic conditions of pH between 3 and 4. It slows down at pH values above 6 and the tan solutions themselves become darker. Vegetable tanning materials can be classified into following groups as shown in figure 7.